Researchers at the University of Maryland have discovered a way to identify and track sulfuric compounds in Earth’s marine environment, opening a path to either refute or support a decades-old hypothesis that our planet can be compared to a singular, self-regulating, living organism — a.k.a. the Gaia theory.
Proposed by scientists James Lovelock and Lynn Margulis in the 70s, the Gaia theory likens Earth to a self-supporting singular life form, similar to a cell. The theory claims that, rather than being merely a stage upon which life exists, life — in all forms — works to actively construct an Earthly environment in which it can thrive.
Although named after the Greek goddess of Earth, the Gaia theory is not so much about mythology or New Age mysticism as it is about biology, chemistry and geology — and how they all interact to make our world suitable for living things.
Once called the Gaia hypothesis, enough scientific cross-disciplinary support has since been discovered that it’s now commonly referred to as a theory.
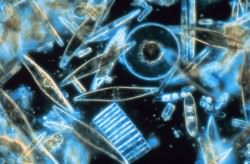
Marine phytoplankton -- like these diatoms -- may produce sulfur compounds that can be transmitted into the air, affecting climate. (NOAA image)
One facet of the Gaia theory is that sulfur compounds would be created by microscopic marine organisms — such as phytoplankton and algae — and these compounds could be transmitted into the air, and eventually (in some form) to the land, thus helping to support a sulfur cycle.
Sulfur is a key element in both organic and inorganic compounds. The tenth most abundant element in the Universe, sulfur is crucial to climate regulation — as well as life as we know it.
In particular, two sulfur compounds – dimethylsulfoniopropionate and its atmospherically-oxidized version, dimethylsulfide — are considered to be likely candidates for the products created by marine life. It’s these two compounds that UMD researcher Harry Oduro, along with geochemist and professor James Farquhar and marine biologist Kathryn Van Alstyne (of Western Washington University) have discovered a way to track across multiple environments, from sea to air to land, allowing scientists to trace which isotopes are coming from what sources.
“What Harry did in this research was to devise a way to isolate and measure the sulfur isotopic composition of these two sulfur compounds,” said Farquhar. “This was a very difficult measurement to do right, and his measurements revealed an unexpected variability in an isotopic signal that appears to be related to the way the sulfur is metabolized.”
The team’s research can be used to measure how the organisms are producing the compounds, under which circumstances and how they are ultimately affecting their — and our — environment in the process.
“The ability to do this could help us answer important climate questions, and ultimately better predict climate changes,” said Farquhar. “And it may even help us to better trace connections between dimethylsulfide emissions and sulfate aerosols, ultimately testing a coupling in the Gaia hypothesis.”
Whether or not Earth can be called a singular — or possibly even sentient — living organism of which all organisms are contributing members thereof may still be up for debate, but it is fairly well-accepted that life can shape and alter its own environment (and in the case of humans, often for the worse.) Research like this can help science determine just how far-reaching those alterations may be.
The study appears in this week’s Online Early Edition of the Proceedings of the National Academy of Sciences (PNAS).
